Pericyclic reaction
In organic chemistry, a pericyclic reaction is a type of chemical reaction between organic compounds. In the case of pericyclic reactions, the transition state of the molecule is a ring (has a cyclic geometry), and the reaction goes forward in a concerted way. Pericyclic reactions are usually rearrangement reactions. The most important groups of pericyclic reactions are:
- Electrocyclic reactions
- Cycloadditions
- Sigmatropic reactions
- Group transfer reactions
- Cheletropic reactions
- Dyotropic reactions

In general, pericyclic reactions are equilibrium processes. However, it is possible to push the reaction in one direction if the product is at a significantly lower energy level. This is applying Le Chatelier's principle to a reaction involving a single molecule.
Many pericyclic reactions have similar stepwise radical processes connected with them. Chemists disagree whether some reactions are pericyclic reactions. For example, it is not definitively known whether the [2+2] cycloaddition mechanism is concerted (or may depend on the reactive system). Many pericyclic reactions have similar reactions that are metal-catalyzed. But these metal-catalyzed reactions are not really pericyclic. The metal catalysts stabilize the reaction intermediates. So the reaction is not concerted, but rather metal-stabilized.
A large photoinduced hydrogen sigmatropic shift was utilized in a corrin synthesis performed by Albert Eschenmoser containing a 16π system.[1]
Due to the principle of microscopic reversibility, there is a parallel set of "retro" pericyclic reactions, which perform the reverse reaction.
Pericyclic reactions in biochemistry
Pericyclic reactions also happen in several biological processes:
- Claisen rearrangement of chorismate to prephenate in almost all prototrophic organisms.
- [1,5]-sigmatropic shift when precorrin-8x is being changed into hydrogenobyrinic acid
- non-enzymatic, photochemical electrocyclic ring opening and a (1,7) sigmatropic hydride shift in vitamin D synthesis.
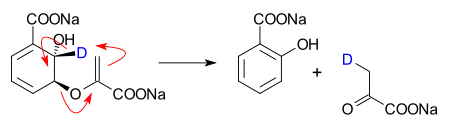
- a conversion of Isochorismate into salicylate and Pyruvate in a catalyzed, true pericyclic reaction.[2][3]