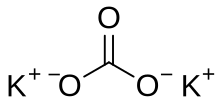
Potassium carbonate
Potassium carbonate (K2CO3) is a white saut, soluble in watter (insoluble in ethanol[2]), which forms a strangly alkaline solution.
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name Potassium carbonate | |
| Ither names Potash, pearl ash | |
| Identifiers | |
CAS Nummer | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| RTECS nummer | TS7750000 |
| UNII | |
| |
SMILES
| |
| Properties | |
| K2CO3 | |
| Molar mass | 138.205 g/mol |
| Appearance | white, hygroscopic solid |
| Density | 2.43 g/cm3 |
| Meltin pynt | 891 °C (1,636 °F; 1,164 K) |
| Bylin pynt | decomposes |
Solubility in watter | 112 g/100 mL (20 °C) 156 g/100 mL (100 °C) |
| Solubility | insoluble in alcohol, acetone |
| Hazards | |
| Main hazards | Irritant |
| R-phrases | R22 R36 R37 R38 |
| NFPA 704 |  0 1 0 |
| Flash pynt | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (Median dose) | 1870 mg/kg (oral, rat)[1] |
| Relatit compoonds | |
Ither anions | Potassium bicarbonate |
Ither cations | Lithium carbonate Sodium carbonate Rubidium carbonate Caesium carbonate |
Except whaur itherwise notit, data are gien for materials in thair staundart state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
🔥 Top keywords: Main PageCarles PuigdemontWikipedia:Contact usSpecial:RecentChangesWikipedia:Commonty YettSalamenceContact lensScots leidFile:Nvidia logo.svgSpecial:SearchFennekinFile:Pizza Hut logo.svgSuzuki EsteemSpecial:MyTalkWarld War IIThe Selkirk GraceWikipedia collogue:Commonty YettMilos RaonicAshley StorrieFile:Starbucks Corporation Logo 2011.svgTemplate:Inflection ofFitbaWikipedia:General disclamationScotlandFile:KFC logo.svgAshley WilliamsWikipaediaFile:Manchester City FC badge.svgFile:Chelsea FC.svgFile:University College London logo.svgFile:Unilever.svgFile:British Airways Logo.svgPatricia NealWikipedia:AbootCategory:Candidates for speedy deletionHelp:ContentsWikipedia:Mercat CrossChief executive officerAnabantha