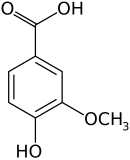
Vanillic acid (4-hydroxy-3-methoxybenzoic acid) is a dihydroxybenzoic acid derivative used as a flavoring agent. It is an oxidized form of vanillin. It is also an intermediate in the production of vanillin from ferulic acid.[2][3]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 4-Hydroxy-3-methoxybenzoic acid | |||
| Other names 4-Hydroxy-m-anisic acid, Vanillate | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.061 | ||
| KEGG | |||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C8H8O4 | |||
| Molar mass | 168.148 g·mol−1 | ||
| Appearance | White to light yellow powder or crystals | ||
| Melting point | 210 to 213 °C (410 to 415 °F; 483 to 486 K) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds | Vanillin, vanillyl alcohol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Occurrence in nature
The highest amount of vanillic acid in plants known so far is found in the root of Angelica sinensis,[4] an herb indigenous to China, which is used in traditional Chinese medicine.
Occurrences in food
Açaí oil, obtained from the fruit of the açaí palm (Euterpe oleracea), is rich in vanillic acid (1616±94 mg/kg).[5] It is one of the main natural phenols in argan oil.[citation needed] It is also found in wine and vinegar.[6]
Metabolism
Vanillic acid is one of the main catechins metabolites found in humans after consumption of green tea infusions.[7]
Synthesis
Vanillic acid can be obtained from the oxidation of vanillin by various oxidizing agents. With Pd/C, NaBH4, and KOH as the oxidizing agent, the conversion was reported to occur in ~89% yield.[8]