Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2. It is a symmetrical molecule with point group D2h. Pyrazine is less basic than pyridine, pyridazine and pyrimidine. It is a "deliquescent crystal or wax-like solid with a pungent, sweet, corn-like, nutty odour".[3]
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Pyrazine[1] | |||
| Other names 1,4-Diazabenzene, p-Diazine, 1,4-Diazine, Paradiazine, Piazine, UN 1325 | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.480 | ||
| EC Number |
| ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C4H4N2 | |||
| Molar mass | 80.09 g/mol | ||
| Appearance | White crystals | ||
| Density | 1.031 g/cm3 | ||
| Melting point | 52 °C (126 °F; 325 K) | ||
| Boiling point | 115 °C (239 °F; 388 K) | ||
| Soluble | |||
| Acidity (pKa) | 0.37[2] (protonated pyrazine) | ||
| -37.6·10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||
  | |||
| Danger | |||
| H228, H315, H319, H335 | |||
| P210, P261, P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 55 °C (131 °F; 328 K) c.c. | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Pyrazine and a variety of alkylpyrazines are flavor and aroma compounds found in baked and roasted goods. Tetramethylpyrazine (also known as ligustrazine) is reported to scavenge superoxide anions and decrease nitric oxide production in human Granulocytes.[4]
Synthesis
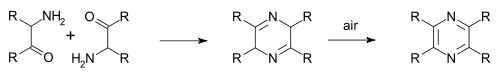
Many methods exist for the organic synthesis of pyrazine and its derivatives. Some of these are among the oldest synthesis reactions still in use.
In the Staedel–Rugheimer pyrazine synthesis (1876), 2-chloroacetophenone is reacted with ammonia to the amino ketone, then condensed and then oxidized to a pyrazine.[5] A variation is the Gutknecht pyrazine synthesis (1879) also based on this selfcondensation, but differing in the way the alpha-ketoamine is synthesised.[6][7]
See also
- Alkylpyrazines
- Methoxypyrazines
- Simple aromatic rings
- Benzene, an analog without the nitrogen atoms
- Pyridazine, an analog with the second nitrogen atom in position 2
- Pyridine, an analog with only one nitrogen atom
- Pyrimidine, an analog with the second nitrogen atom in position 3
- Piperazine, the saturated analog