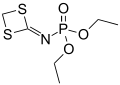
In organophosphorus chemistry, phosphoramidates (sometimes also called amidophosphates) are a class of phosphorus compounds structurally related to phosphates (or organophosphates) via the substitution of an −O− group for an amine group (−N−). They are derivatives of phosphoramidic acids, which possess the structure O=P(OH)(NR2)2 or O=P(OH)2(NR2).
A phosphorodiamidate is a phosphate that has two of its hydroxyl (−OH) groups substituted by amine (NR2) groups to give a species with the general formula O=P(OH)(NH2)2. The substitution of all three OH groups gives the phosphoric triamides (O=P(NR2)3), which are commonly referred to as phosphoramides.[1]
Synthesis
In the Stokes method, phosphoramidates are synthesized from phosphorus oxychloride. The compound reacts with phenol to form a chlorophosphonate ester or diester, depending on stoichiometry. The remaining chlorine substituents then react with an amine compound to give the phosphoramidate.[2]
Examples
Two examples of natural phosphoramidates are phosphocreatine and the phosphoramidate formed when histidine residues in histidine kinases are phosphorylated.[3]An example of a phosphorodiamidate is morpholino which is used in molecular biology.