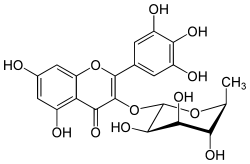
Myricitrin is a plant compound, the 3-O-α-L-rhamnopyranoside of myricetin.[1]
 | |
| Names | |
|---|---|
| IUPAC name 3′,4′,5,5′,7-Pentahydroxy-3-(α-L-rhamnopyranosyloxy)flavone | |
| Systematic IUPAC name 5,7-Dihydroxy-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}-2-(3,4,5-trihydroxyphenyl)-4H-1-benzopyran-4-one | |
| Other names Myricitroside Myricitrine Myricetrin Myricetol 3-rhamnoside Myricetin 3-O-rhamnoside Myricetin 3-rhamnoside | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.038.036 |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| Properties | |
| C21H20O12 | |
| Molar mass | 464.37 g/mol |
| Density | 1.882 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Occurrences
It can be isolated from the root bark of Myrica cerifera (Bayberry, a small tree native to North America), in Myrica esculenta, in Nymphaea lotus[2] and N. odorata, in Chrysobalanus icaco[3] and in Polygonum aviculare.[4]
Myricitrin is used by several beetle species in their communication system.[5] These include Plagioderma versicolora, Agelastica coerulea, Atrachya menetrisi, Altica nipponica, Altica oleracea, Gastrolina depressa.
Health effect
Myricitrin is a nitric oxide and protein kinase C inhibitor and exhibits antipsychotic-like and anxiolytic-like effects in animal models of psychosis and anxiety respectively.[6]
References
🔥 Top keywords: Main PageSpecial:SearchPage 3Wikipedia:Featured picturesHouse of the DragonUEFA Euro 2024Bryson DeChambeauJuneteenthInside Out 2Eid al-AdhaCleopatraDeaths in 2024Merrily We Roll Along (musical)Jonathan GroffJude Bellingham.xxx77th Tony AwardsBridgertonGary PlauchéKylian MbappéDaniel RadcliffeUEFA European Championship2024 ICC Men's T20 World CupUnit 731The Boys (TV series)Rory McIlroyN'Golo KantéUEFA Euro 2020YouTubeRomelu LukakuOpinion polling for the 2024 United Kingdom general electionThe Boys season 4Romania national football teamNicola CoughlanStereophonic (play)Gene WilderErin DarkeAntoine GriezmannProject 2025