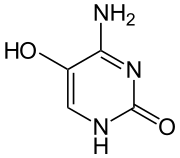
5-Hydroxycytosine is an oxidized form of cytosine that is associated with an increased frequency of C to T transition mutations, with some C to G transversions.[1] It does not distort the DNA molecule and is readily bypassed by replicative DNA polymerases.[2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name 4-Amino-5-hydroxypyrimidin-2(1H)-one | |
| Other names 4-Amino-5-hydroxypyrimidine-2-one | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C4H5N3O2 | |
| Molar mass | 127.103 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
It has been shown in vitro to miscode for adenine.
5-hydroxycytosine is imperative for parallel DNA triplex formation, explaining why parallel triplexes form only at pH 6 and below.
References
🔥 Top keywords: Main PageSpecial:SearchPage 3Wikipedia:Featured picturesHouse of the DragonUEFA Euro 2024Bryson DeChambeauJuneteenthInside Out 2Eid al-AdhaCleopatraDeaths in 2024Merrily We Roll Along (musical)Jonathan GroffJude Bellingham.xxx77th Tony AwardsBridgertonGary PlauchéKylian MbappéDaniel RadcliffeUEFA European Championship2024 ICC Men's T20 World CupUnit 731The Boys (TV series)Rory McIlroyN'Golo KantéUEFA Euro 2020YouTubeRomelu LukakuOpinion polling for the 2024 United Kingdom general electionThe Boys season 4Romania national football teamNicola CoughlanStereophonic (play)Gene WilderErin DarkeAntoine GriezmannProject 2025